- Date
- 16th June 2020
- Categories
By Dr Nigel Monk, Dr Richard Blanchard and Dr John Barton (CREST, Loughborough University).
Reducing the costs of cooking is one of the enablers to improve access to MECS which can be tackled in a number of complementary ways such as reducing appliance purchase price or improving efficiency in use. Electric pressure cookers (EPCs) already compare favourably against conventional stoves – and very favourably against biomass cooking – in terms of energy consumption, but commercial product specifications always include trade-offs.
One of MECS’ ongoing mandates for appliances that have evolved to satisfy a first-world set of norms is to critically examine embedded assumptions against the needs of the global south to provide innovative solutions with a relevant product emphasis.
One prevailing belief at the outset of the MECS’ programme was that adding insulation to an EPC would reduce energy consumption further. For traditional pans, the ‘wonder bag’, a cloth bag made from quilted insulation with a tie-drawn opening, is very successful at keeping a pan of food hot enough to remain self-cooking for a considerable time, significantly reducing the energy required to cook beans, for example. Could an EPC be similarly improved? Although the wonder bag has attractive benefits, it needs to be fully enclosing to be effective.
Unfortunately, enclosing an entire EPC in an insulating cover would not be safe; the function of the pressure-limiting steam valve would be compromised. Instead, it should be possible to install a layer of insulation internally between the chassis and the outer skin, including within the lid. The Aobosi 6 litre cooker in figure 1 has an additional, plastic outer cover above the metal pressure-vessel lid which creates an air gap of c.1.5 cm. This space made the Aobosi an ideal test appliance.
Experiment.
Two identical cookers were tested simultaneously, one with added insulation, one as bought, so that mains voltage and environmental temperature variations did not affect the comparison. A report of the results is available here.
To test the theory, using the best insulation would allow the best chance of producing the most benefit. It is widely known that the aerogel class of materials offers the greatest insulation resistance available. Aspen Pyrogel XTE™ is a compressed mineral wool impregnated with aerogel which is naturally resistant to absorbing moisture. For the Aobosi, the most that could be applied was a single 10mm layer top and bottom and a double layer around the sides requiring roughly 1m2 at a cost of c.£80, see figure 2.
An extensive series of water boiling tests were undertaken. Water boiling tests are the most effective tool to accurately assess appliance efficiency and behaviour and avoid the issue of wasted food from other types of tests. The tests were repeated at different volumes while measuring internal water temperature and 8 external temperatures.

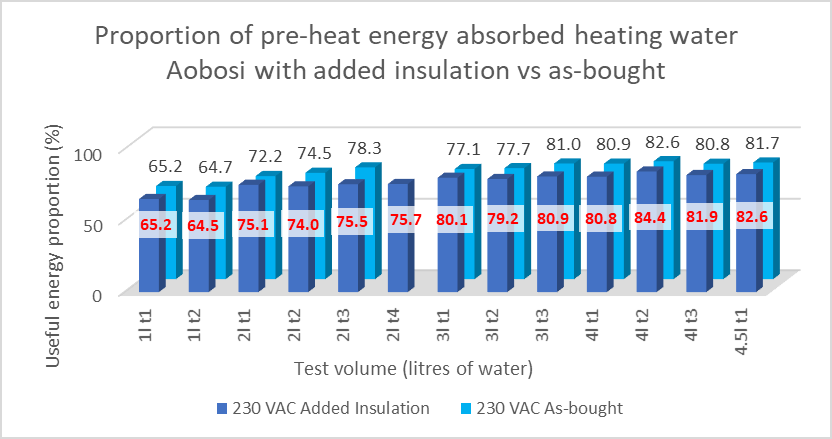
The parasitic thermal mass of a typical EPC calculated from empirical energy consumption data is c. 80 Wh. This represents the total energy absorbed by the EPC and insulation during the pre-heat phase. The Pyrogel XTE material added 0.16 Wh/K thermal mass, equivalent to 0.136 litres of water. Therefore, at a simplistic level, the Pyrogel XTE adds between 3.7% and 1.2% overall increase in thermal mass for a 1 litre or 5 litres volume of water respectively. From figure 3, this can be seen to fall within experimental variations between repeated tests.
Therefore, we would expect the energy and time required to raise the EPC and water temperature to be increased by a small amount, and the average energy input rate during cooking to be reduced by an amount. The former is best measured via ‘proportion of pre-heat energy absorbed heating the water’ where any increase in parasitic thermal mass will reduce this basic efficiency. The reduction in cooking energy required is directly measured via ‘average energy input rate during cooking’.
Pre-heating phase.
During the pre-heating phase, energy lost to the environment comprises 4% of the total preheat energy input, for all volumes of water. Adding the Pyrogel XTE reduced the loss by 22% (see figure 4) producing an overall improvement of 0.9%. The improvement is offset by the larger increase in parasitic thermal mass. During the tests, there were no visible differences in the pre-heat phase between the two cookers that could not have been ascribed to experimental variation, see figure 3.
Cooking phase.
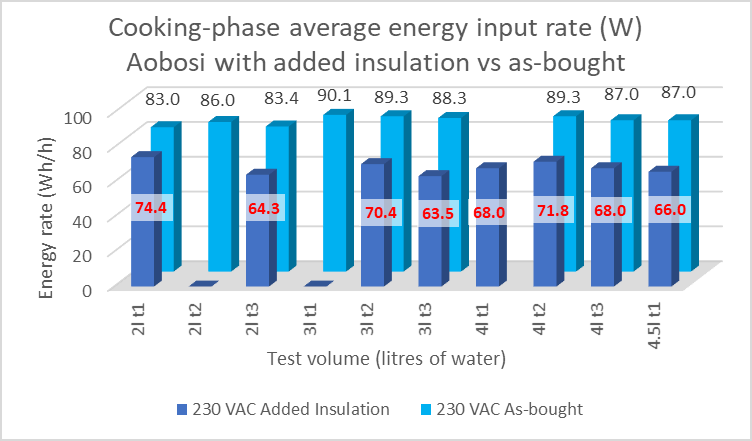
During the cooking phase, the 22% saving will apply to the whole energy input, see figure 4. However, because the EPC power is cycling on and off at a duty ratio c.10%, absolute saving is the product of these at c.2% of the nominal power rating.
The net effect is thus, for 1 hour of cooking, independent of the water volume, the insulation will save c.20 Wh of energy.
Discussion.
Installing the Pyrogel displaces 4.75 litres or 6g of air with thermal mass 1.6×10-3 Wh/K and thermal conductivity = 0.59 W/K. However, the insulation is calculated to conduct 0.46 W/K, a reduction of 22%. The thermal conductivity reduction is relatively low because air is also an excellent insulant provided convection is minimal.
The measured energy input rates during cooking confirmed the 22% reduction in heat loss rate. This can save around 20 Wh per hour of cooking duration. This is a small amount and the benefits of extra insulation in an already efficient device can be seen to be negligible. The results confirmed negligible difference in the energy performance of the two EPCs.
The net effect of this can be seen in the thermographic images in figure 5. Although there are obvious differences, the external temperatures for both EPC’s are far below the internal temperature of the water except at the junction of the lid and body, where the ‘moat’ forms a thermal bridge. Adding insulation within the cooker will never address this issue, which could require re-engineering of the component. A lid that covered the joint might be an alternative approach and should not require a wholesale redesign of the EPC per se.
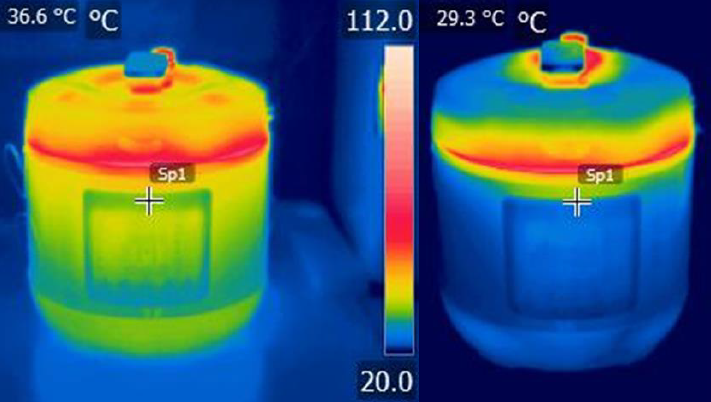
Although thermal insulation was not greatly enhanced in the case of the Aobosi, a less-well designed EPC may benefit more. In particular, an EPC with a single layer stainless steel lid, where surface temperatures have been recorded as high as 105°C, might benefit from a top-half cover to insulate the moat and avoid scalds. However, the cover will need to be tailored to a specific EPC to provide holes to avoid the various steam vents in the lid. The cover could be made in country from natural materials.
It is clear that enclosing the cooking pan, whether in an EPC, multicooker or wonderbag, does reduce energy consumption compared to an unprotected pan. Although air is not normally thought of as an insulating material, when air is trapped such as in the gap between the pan and chassis so that no replacement and limited circulation takes place, this helps trap the heat. In contrast, on a single walled pan, air is heated by contact with the pan and energy is lost as the hot air convects away to be replaced with fresh cool air.